A golden age for oncology
I've often considered what would be a realistic effect to power an overall survival (OS) trial on. So I thought I’d go look in a few labels to see what’s been observed and then I thought why don’t I go look at all the labels in the last 20 years, like you do!
As a by product this confirmed we’re in the middle of what looks to be a golden age for oncology – the number of drugs that have a labelled OS HR ≤0.75 in the last 5 years is more than the previous 15 years put together.
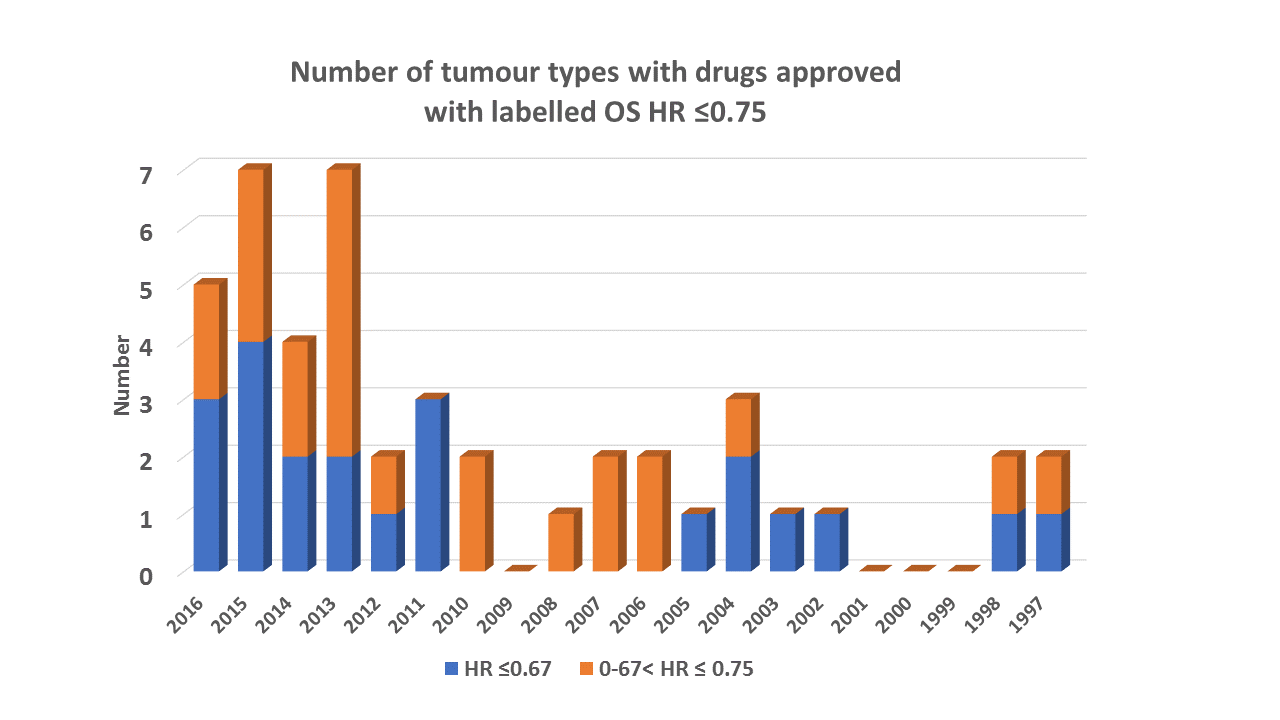
Only advanced cancer indications were considered. A drug was counted a maximum of once per tumour type from US labels and only if it was statistically significant and the HR was less than the relevant threshold. Drugs are assigned to the year in which they were first approved for that tumour type. This may actually mean recent years may turn out to be undercounted.
So how does this inform the sizing of PIII trials? Well if you have prior randomised data on OS, or an intermediate that predicts OS reasonably well, or your drug is in the same class as a predecessor then you’ll have some drug specific data to go on – often that’s not the case though.
It’s been reported that over 300 PIII programmes were started in oncology in the last 10 years. Given that only 15 times (<5%) has a HR < 0.67 been observed for a new drug in a given disease, it would seem highly optimistic to size a trial on a HR=0.67. If this optimism is misplaced, yet the agent has an impressive HR of 0.75, there becomes an uncomfortable chance (35-50%) of failing. A better strategy would be to size the trial on a more realistic 0.75 HR and introduce an interim analysis which would have a good chance (>50%) of hitting the interim boundary, if in fact the optimism was justified.
Alternative Design Strategies

The introduction of crossover will likely have played a role here and I wonder whether part of the recent upturn is that recent classes of therapy often haven’t produced especially high response rate in early phase development and trialists have then felt able to run randomised trials without crossover.